Low hydrogen electrodes are SMAW electrodes with very low moisture content. Example of such low hydrogen electrode is E7018.
We know that moisture i.e. water is made up of hydrogen and oxygen items (H2O) i.e. 2 atoms of hydrogen and one atom of oxygen makes up a water molecule.
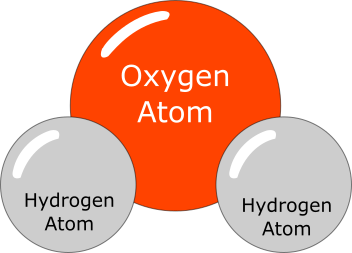
A typical arc welding process involves intense heat and electric current along with electric arc. This environment breaks some of the water molecules into individual parts i.e. hydrogen atoms and oxygen atoms.
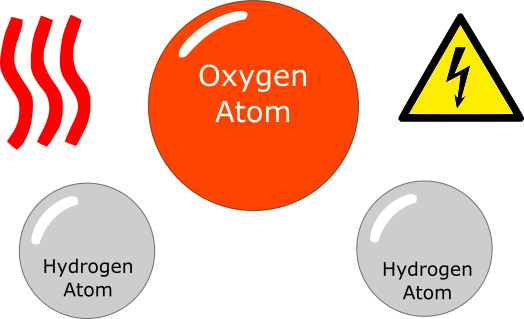
Hydrogen atoms are very small in size and can diffuse through steel metal, specially when it is very hot as the spacing between grains of metal increases when it is hot.
When metal cools down, hydrogen atoms recombine with each other to form hydrogen molecules i.e. H2. It can also react with carbon inside steel to form methane gas.
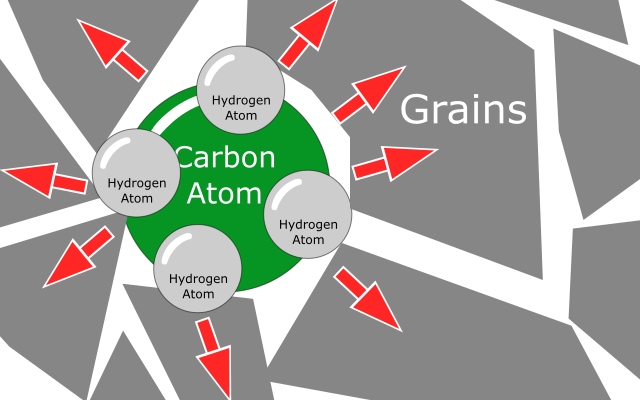
These molecules of hydrogen and methane are bigger in size than hydrogen atom. Also at this time, spacing between grains of metal decreases. These two things causes extreme pressure of hydrogen and methane molecules on grain boundaries, causing metal to crack.

Where they are used
High strength steels such as armor steel or those used in oil and gas industry are most affected by hydrogen induced cracking.
Low Hydrogen Electrode Specifications
Low hydrogen electrodes are specified in AWS A5.1 filler metal specification 3. This specification lists several electrode classifications with “low hydrogen” coatings.
Moisture Content
These classifications must have a coating moisture level of less than 0.6% when tested at 1800 °F (980 °C), according to AWS A5.1. This moisture level corresponds to a relatively low diffusible hydrogen level in the deposited weld metal, typically less than 16 mL/100g.
For example, AWS A4.3, Standard Methods for Determination of Diffusible Hydrogen4, shows that when E7018 is welded at 70 °F and 60% relative humidity a 0.6% coating moisture equates to approximately 12 mL/100g of diffusible hydrogen.
Many of today’s E7018 products have actual coating moisture content levels much lower than the maximum of 0.6% in the as-received condition.
Other factors to be taken into consideration
only usinglow hydrogen electrode doe not provide guarantee of eliminating problems related to hydrogen during or after welding. Several other factors can influence the diffusible hydrogen level and the potential for cracking.
- Base metal surface condition (contamination from oils, grease, dirt, moisture, acid, rust and other hydrogen containing materials can increase hydrogen levels)
- Relative atmospheric humidity (humid conditions generally lead to higher hydrogen levels).
- Welding shielding gas (higher moisture content results in higher hydrogen levels).
- Consumable storage conditions (improper or prolonged storage can lead to higher hydrogen levels).
- Welding procedures (electrical stickout, arc voltage, wire feed speed and other parameters can influence diffusible hydrogen)
